Sample Tutorial 3: The Partial Demyelination Tutorial
The axon in this tutorial is bare on its left half and myelinated on its right half. Intracellular electrodes record the voltage in the middle of the bare portion and at two nodes in the myelinated region. An action potential can be triggered at either end of the axon and its propagation displayed as a moving graph.
This tutorial should help the user understand how action potentials propagate, how they spread out in myelinated axon, and why they struggle to propagate from a myelinated portion of the axon into a demyelinated region.
- In the actual tutorial on the CD, clicking the START THE SIMULATION button would bring up the control panels and plotting graphs.
- Thumbnails along the left side of the text below can be clicked to show screenshots from the tutorial in progress.
- Note that neither the tutorial nor the hyperlinks are operative here.
Partial Demyelination
The Problem in Multiple Sclerosis
This tutorial simulates the condition of multiple sclerosis.
This tutorial simulates an action potential in myelinated nerve attempting to propagate through a demyelinated (bare) region, as in multiple sclerosis (MS). In this disease, axons become demyelinated in a patchy and unpredictable fashion, leading to a host of sensory and motor symptoms.
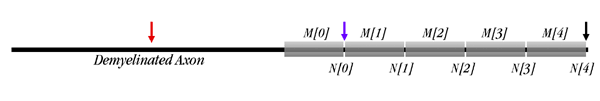
The preparation is demyelinated on the left half.
The axon is 10,000 μm long, as shown in the diagram below. The right half is myelinated, with 5 node/myelinated internode pairs; the left half is demyelinated. You can change parameters both for the bare half and for the myelinated half. In the tutorial you will first insert a stimulating electrode into the left end of the bare axon, then move it to the right end of the myelinated region (node[4]).
How are channels distributed in the membrane of a demyelinated axon?
Ion channel densities have been assigned on the basis of experimental observations. The densities in the nodes are ten-fold greater than those in squid axon. The bare axon has been assigned the normal density of channels in unmyelinated nerve, distributed uniformly. The distribution of channels in demyelinated axon is only partially understood at present and depends on how long the axon has been demyelinated.

Goals of this Tutorial
-
To observe the shape of the action potential as it travels from the demyelinated region of the axon into the myelinated region
-
To observe the features of the action potential as it tries to invade the bare axon from the myelinated region
-
To observe how changes in the ion conductances in the bare axon and also in temperature affect the ability of the action potential to invade the bare axon from the myelinated portion
Description of the Panels and Windows Customized for this Tutorial
-
AssumptionsThis tutorial assumes that you are now familiar with the following panels and manipulations:
- The function of the buttons within the P&G Manager and Run Control panels
- Running simulations by clicking Reset and Run (R&R) or clicking Reset and then the "Continue for (ms)" button in the Run Control panel
- Inserting stimulating electrodes by launching the Stimulus Control panel and controlling the delay, amplitude, and duration of the stimulus
- Storing, erasing, re-sizing, and measuring values on traces in plotting windows using the right mouse button features
- Using the field editor or the arrows box to change values in the parameters panels
If this tutorial is your introduction to Neurons in Action, we suggest that you familiarize yourself with the panels and operations listed here by clicking their links. -
The stimulating electrodesTwo "Stimulus Control" buttons are available in the P&G Manager, one to put the stimulating electrode (Stimulus 'Trode) in the left end of the bare axon (brought up initially) and the other to put it in the right end (node[4]) of the myelinated segment. Clicking either of these buttons will bring up separate Stimulus Control panels.Although the electrode may be moved after insertion as usual by clicking on the line representing the axon in this panel, it is preferable to switch locations using the Stimulus Control buttons because the amplitude of the current stimulus needs to be different in the bare axon and in the myelinated segment.
-
The graphsIn the Voltage-vs-Time graph, the traces are color-coded to the three recording sites shown in the diagram above and on the axis of the Voltage-vs-Space graph:
- At the center of the bare half of the axon
- At node[0]
- At node[4]
Experiments and Observations
Observe impulses traveling in partially demyelinated axons.
-
Stimulate the left end of the demyelinated (bare) axon.
-
Observe the location of the stimulating electrode.The Stimulus Control panel that comes up upon launching the simulation shows a stimulating electrode in the left end of the bare half of the axon. The amplitude of the current pulse has been set to a large enough value to evoke an action potential in the bare portion.
-
Run the simulation (press R&R)The impulse will be initiated at the far-left end of the demyelinated region and travel to the right into the myelinated region.
-
Relate the Voltage-vs-Time recordings to the Voltage-vs-Space movie.As you did in the Unmyelinated Axon tutorial, watch the voltage at a point in the Voltage-vs-Space graph as it moves up and down in time. If you pick a point where a recording electrode is located (for example, in the middle of the bare axon), you can compare it to the trace in the Voltage-vs-Time graph.To slow down the process, click the "Continue for (ms)" button in the Run Control panel to play and pause the movie repeatedly. Or click the "Stop" button to stop the movie at any time.
-
Stop the impulse in the bare axon.
 Make certain you understand the shape of the action potential in both plots. For example, which phase is the rising phase in the Voltage-vs-Time plot and in the Voltage-vs-Space plot? Can you explain the shape of the rising phase in the Voltage-vs-Space plot? If you understand the shape, you probably have a good understanding of how the impulse propagates.
Make certain you understand the shape of the action potential in both plots. For example, which phase is the rising phase in the Voltage-vs-Time plot and in the Voltage-vs-Space plot? Can you explain the shape of the rising phase in the Voltage-vs-Space plot? If you understand the shape, you probably have a good understanding of how the impulse propagates.
-
Close the Stimulus 'Trode in Bare Axon panel.This action will remove the stimulating electrode. You will next insert it at the other end of the axon. Although you can move the electrode to the other end of the axon by clicking on the line, the amplitude of the stimulus will then be inappropriately large for stimulating the myelinated segment, as mentioned above.
Attention! Next you will put the stimulating electrode in node[4]. It is necessary to close the Stimulus 'Trode in Bare Axon panel to avoid generating impulses at both ends of the axon. -
-
Reverse the direction of stimulation: Excite the myelinated region.
 Press Stimulus 'Trode in Node[4] to insert the stimulating electrode at the rightmost node of the myelinated region. Run the simulation. What happens to the impulse at the junction between the myelinated and bare axon? Explain your observations. Relate what you see in the Voltage-vs-Space movie to your recordings with the three electrodes in the Voltage-vs-Time graph.
Press Stimulus 'Trode in Node[4] to insert the stimulating electrode at the rightmost node of the myelinated region. Run the simulation. What happens to the impulse at the junction between the myelinated and bare axon? Explain your observations. Relate what you see in the Voltage-vs-Space movie to your recordings with the three electrodes in the Voltage-vs-Time graph.
A change in temperature is known to improve the condition of multiple sclerosis patients. Investigate the basis for this phenomenon.
-
Make an educated guess.
Hint: What change will enable the action potential in the myelinated segment to supply more current to the bare axon? -
Test your hypothesis.
Change the temperature (in the Run Control panel). Run the simulation to see if impulse invasion of the demyelinated region improves or worsens. A detailed discussion of the connection between temperature, threshold, and impulse propagation is available.(Although the temperature range in which you are experimenting is appropriate for frog axon and not for humans, the principle is the same for both species.) -
Question:
What is the smallest change in temperature required to produce any difference you observe? Your observations have a clinical correlation in the Uhthoff phenomenon. -
Observe impulse resurgence in the myelinated axon.
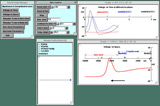 Note that at a certain critical temperature the conditions are just right for the impulse to resurge in the myelinated region; in the Voltage-vs-Time graph you should be able to see two action potential peaks at node[4] (black trace).
Note that at a certain critical temperature the conditions are just right for the impulse to resurge in the myelinated region; in the Voltage-vs-Time graph you should be able to see two action potential peaks at node[4] (black trace).
-
Restore the temperature to the default value of 25.2 °C.
What changes in axon parameters will permit the impulse to invade the bare region?
-
Launch the Bare Axon Parameters panel.
In this panel you can alter parameters of the demyelinated portion of the axon. When you have changed a parameter, remember to reset it before changing another one. You can experiment with changes that will promote impulse invasion of the bare axon.-
Change the density of functional K channels.
 For a hint, read a quote from the National Institutes of Health web page entitled "Therapy to improve nerve impulse conduction."
For a hint, read a quote from the National Institutes of Health web page entitled "Therapy to improve nerve impulse conduction."
-
Change the density of functional Na channels.By how much must you change the density to enable invasion of the bare axon?
-
Change the diameter of the bare axon.In what direction would you expect a change to facilitate impulse invasion?Hint: Changing the diameter of the bare axon changes the membrane area and thus the capacitance that the current from the myelinated axon is required to charge.
-
Prepare for the next experiments.Be certain to restore all bare axon parameters to their default values. You can close the Bare Axon Parameters panel or leave it open.
-
-
Change parameters of the myelinated axon.
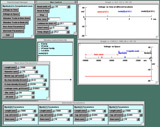 Click the "Internode Parameters" button. Four menus will come up in a "tray" for four of the five internodes (M[0] through M[3] on the diagram above). The far right internode, M[4], is left out. You can adjust the length of each internode, its degree of myelination (the capacitance, which is 1 μF/cm2 divided by the number of wraps), and the inside diameter of the axon (the diameter of the axon without its wrapping).
Click the "Internode Parameters" button. Four menus will come up in a "tray" for four of the five internodes (M[0] through M[3] on the diagram above). The far right internode, M[4], is left out. You can adjust the length of each internode, its degree of myelination (the capacitance, which is 1 μF/cm2 divided by the number of wraps), and the inside diameter of the axon (the diameter of the axon without its wrapping).
-
Experiment with the internode M[0].What change in the parameters of this adjacent internode will increase the longitudinal current into the bare axon and cause the action potential to propagate there?
- Questions: What if you change the length of this myelinated segment? Should it be longer or shorter to supply more current to the bare axon? How much change is needed?
- Question: What if you change the degree of myelination of this segment by changing the capacitance?
- Question: Will changing the diameter of this one segment have an effect? Hint: Any change that increases the longitudinal current supplied to the bare region of axon from the myelinated region will assist the struggling action potential to become regenerative.
-
Change parameters of the other internodes.How crucial is the adjacent internode compared to the more remote internodes? Experiment in a similar fashion with the other three internodes.
-